m-CPBA
(m-Chloroperoxybenzoic Acid)
Other Names:
m-Chloroperbenzoic acid
3-Chloroperoxybenzoic acid
3-Chloroperbenzoic acid
General Information:
Structure:
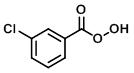
CAS Number: 937-14-4
Molecular Weight: 172.57 g/mol
Appearance: White powder
Melting Point: 92-94 C
Acidity (pKa): 7.57
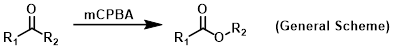
m-CPBA is a peroxycarboxylic acid that is commonly used as an oxidizing agent. It is usually available in <77% form because pure m-CPBA is shock sensitive (potentially explosive). It usually consists of ~10% m-chlorobenzoic acid and the remainder is H2O.
Common Uses:
Reagent for the oxidation of sulfides to sulfoxides
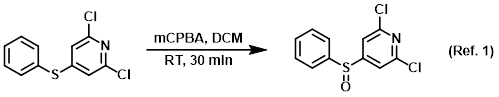
Procedure excerpt:
To a solution of the SM (2.38 g, 9.29 mmol) in DCM (25 mL) was added dropwise a solution of mCPBA (77%, 2.08 g, 9.29 mmol) in DCM (25 mL). The reaction mixture . . .
Reagent for the oxidation of sulfides to sulfones
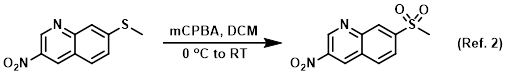
Procedure excerpt:
To solution of the SM (141 mg, 0.6 mmol) in DCM (6 mL) at 0 C was added mCPBA (221 mg, 1.3 mmol) in DCM (3 mL). After warming to RT a white precipitate . . .
Reagent for the oxidation of alkenes to epoxides
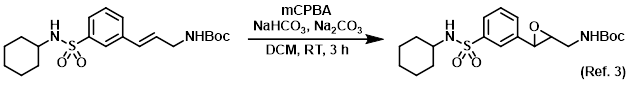
Procedure excerpt:
To a solution of the SM (0.48 g, 1.217 mmol) in DCM was added mCPBA (77%, 0.72 g, 3.2 mmol), followed by NaHCO3 (0.24 g, 2.86 mmol) and Na2CO3 (0.24 g, 2.27 mmol). . . .
Reagent for Baeyer-Villiger oxidation
Reagent for the oxidation of nitrogen-containing compounds
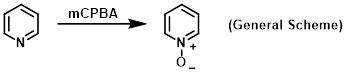
Safety:
Pure m-CPBA can be detonated by shock or sparks.
References:
1) Patent Reference: WO2016001341, page 101, ![]() (9.1 MB)
(9.1 MB)
2) Patent Reference: WO2007084786, page 113, ![]() (9.4 MB)
(9.4 MB)
3) UK Pat App GB2463151A, page 179
4) Wikipedia: meta-Chloroperoxybenzoic acid (link)
5) www.sigmaaldrich.com: 3-Chloroperbenzoic acid (link)
6) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
