Benzyl Bromide
Other Names:
Bromomethylbenzene
α-Bromotoluene
General Information:
Structure:
![]()
CAS Number: 100-39-0
Molecular Weight: 171.03 g/mol
Appearance: Colorless liquid
Melting Point: -4 C
Boiling Point: 201 C
Density: 1.438 g/mL at 25 C
Benzyl bromide is typically used for installing benzyl protecting groups on alcohols and amines. Benzyl bromide is a strong lachrymator and should be handled in a fume hood. Concentrating reaction mixtures on rotovaps that are not in a fume hood can lead to enough exposure for the irritating effects to be noticed. Purifying crude reaction material on automated purification machines outside a fume hood can also provide sufficient exposure to notice the effects (irritated eyes, coughing, etc.).
Common Uses:
Reagent in the benzyl protection of amines
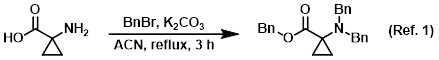
Procedure excerpt:
To a solution of the SM (10.0 g, 99.0 mmol) in ACN (300 mL) was added K2CO3 (39.4 g, 285 mmol) and BnBr (41.0 mL, 346.0 mmol). The reaction mixture . . .
Reagent in the benzyl protection of alcohols
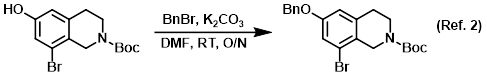
Procedure excerpt:
A solution of the SM (22.5 g, 68.6 mmol) and K2CO3 (28.4 g, 205.7 mmol) in DMF (200 mL) was stirred at RT for 2 h. The mixture was then treated with BnBr . . .
Safety:
Benzyl bromide is a severe irritant to the eyes, mucous membranes (mouth, throat, lungs, etc.), and skin. Exposure can cause intense pain and irritation of the eyes, nose, and throat similar to the effects of tear gas. Difficulty with breathing may result temporarily. Acute exposure may be fatal. Permanent eye damage can result from a large exposure. Benzyl bromide is a combustible liquid.
References:
1) Patent Reference: WO2014149164, page 232, ![]() (23.7 MB)
(23.7 MB)
2) Patent Reference: WO2016014463, page 105, ![]() (6.7 MB)
(6.7 MB)
3) Wikipedia: Benzyl bromide (link)
4) www.sigmaaldrich.com: Benzyl bromide (link)
5) www.alfa.com: A13535 Benzyl bromide, 99% (link)
6) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis; Activating Agents and Protecting Groups
