Substitution (Mesylate)
(Aromatic Alcohols)
Examples:
Example 1
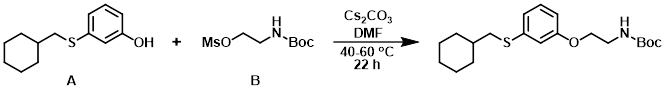
The crude mesylate (B) (0.910 g, 3.80 mmol) was added to a stirred mixture of the phenol (A) (0.745 g, 3.35 mmol), Cs2CO3 (1.373 g, 4.21 mmol), and dry DMF. The reaction mixture was stirred at 60 C for 2 h, then 40 C for 20 h. The mixture was diluted with H2O and extracted with EtOAc (2x). The combined organics were washed with brine, dried (MgSO4), concentrated, and purified by flash chromatography (10-40% EtOAc/hexane) to provide a mixture of the product and unreacted phenol (3.5:1 molar) as a colorless oil. The material was taken to the next step without further purification. [0.874 g, 71%] [UK Pat App GB2463151A, page 127]
