SNAr (Br)
[Aliphatic Amines (1o)]
Examples:
Example 1
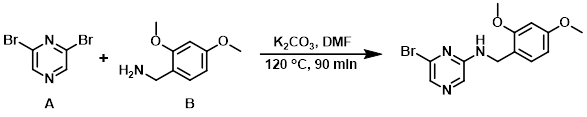
A mixture of the amine (B) (668 mg, 4.0 mmol), K2CO3 (1.1 g, 8.0 mmol), and the aryl bromide (A) (1.42 g, 6.0 mmol) in DMF (10 mL) was stirred at 120 C for 90 min. After cooling to RT, the mixture was poured into H2O (60 mL) and extracted with EtOAc (2 x 60 mL). The combined organics were washed with brine (60 mL), dried (Na2SO4), and concentrated. The resulting residue was purified by silica gel chromatography (2:1 PE/EtOAc) to provide the product as a yellow solid. [300 mg, 23%]
[Patent Reference: WO2016011390, page 154, ![]() (20.2 MB)]
(20.2 MB)]
