Diethyl Ether
Other Names:
Ether
Ethyl ether
Ethoxyethane
General Information:
Structure:
![]()
CAS Number: 60-29-7
Molecular Weight: 74.12 g/mol
Appearance: Colorless liquid
Melting Point: -116 C
Boiling Point: 35 C
Density: 0.706 g/mL
Common Uses:
Solvent for reactions
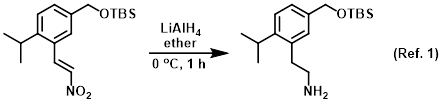
Procedure excerpt:
LiAlH4 (283 mg, 7.45 mmol) was added to a stirred solution of the SM (1.0 g, 2.98 mmol) in ether (30 mL) at 0 C. The reaction mixture was stirred at 0 C for 1 h. To the mixture . . .
Solvent for extractions
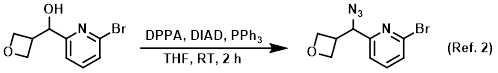
Procedure excerpt:
. . . The mixture was diluted with ether and washed with sat aq NaHCO3, brine, dried (MgSO4), and concentrated . . .
Solvent for trituration
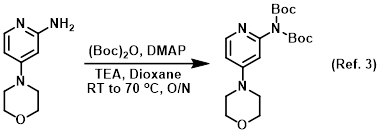
Procedure excerpt:
. . . The resulting residue was triturated with diethyl ether, filtered, and further washed with diethyl ether to provide the pure product . . .
Safety:
Diethyl ether is highly volatile and its vapors are extremely flammable. The fact that diethyl ether vapors are denser than air makes it even more dangerous because the vapors tend to 'hang around' longer. Diethyl ether also tends to slowly form explosive peroxides over time. Bottles that have been open typically should be checked every three months for peroxide formation. After one year it is typically recommended to dispose of bottles of diethyl ether that have been opened. Diethyl ether also has anesthetic properties and has potential as a recreational drug due to its euphoric properties upon exposure.
References:
1) Patent Reference: WO2015144799, page 341, ![]() (18.8 MB)
(18.8 MB)
2) Patent Reference: WO2016011390, page 412, ![]() (20.2 MB)
(20.2 MB)
3) Patent Reference: WO2010038081, page 275, ![]() (33.8 MB)
(33.8 MB)
4) Wikipedia: Diethyl ether (link)
5) www.sigmaaldrich.com: Diethyl ether (link)
