CDI
(Carbonyldiimidazole)
Other Names:
1,1'-carbonyldiimidazole
N,N'-carbonyldiimidazole
General Information:
Structure:
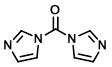
CAS Number: 530-62-1
Molecular Weight: 162.15 g/mol
Appearance: White cystalline solid
Melting Point: 116-118 C
Carbonyl diimidazole (CDI) is a useful alternative to highly toxic phosgene. CDI is similar to phosgene in its synthetic utility but is easier to handle since it exists as a solid. CDI is moisture sensitive and reacts readily with H2O to evolve CO2. CDI can be used for the activation of carboxylic acids. These activated carboxylic acids can be reacted with nucleophiles to make product that such as amides, esters, ureas, and carbamates. CDI reactions often don’t require a base because the liberated imidazole anion acts a base.
Common Uses:
Reagent for amide coupling reactions
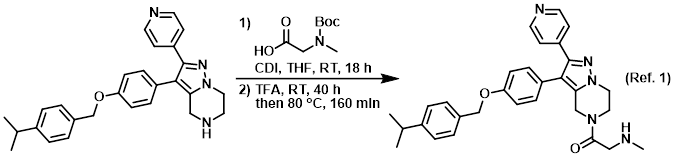
Procedure excerpt:
The acid (152 mg, 0.801 mmol) was added to a solution of CDI (130 mg, 0.801 mmol) in dry THF (1 mL) at RT under N2. The mixture was stirred for 90 min at RT, after which time . . .
Reagent for urea formation reactions
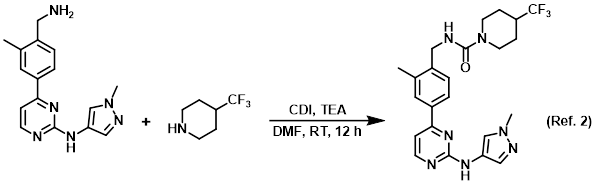
Procedure excerpt:
. . . was added TEA (48 mg, 0.48 mmol). The resulting mixture was stirred at RT for 10 min, after which time was added CDI (39 mg, 0.24 mmol). The mixture was stirred . . .
Safety:
Carbonyldiimidazole (CDI) is moisture sensitive and reacts readily with H2O to give off CO2.
References:
1) Patent Reference: WO2015144799, page 287, ![]() (18.8 MB)
(18.8 MB)
2) Patent Reference: WO2015089337, page 98, ![]() (17.5 MB)
(17.5 MB)
3) Wikipedia: Carbonyldiimidazole (link)
4) www.sigmaaldrich.com: CDI (link)
5) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
6) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
