Ammonium Acetate
Other Names:
Ammonium ethanoate
Acetic acid ammonium salt
General Information:
Structure:
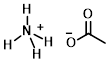
CAS Number: 631-61-8
Molecular Weight: 77.08 g/mol
Appearance: White solid
Chemical Formula: NH4CH3CO2
Melting Point: 113 C
Ammonium acetate (NH4OAc) is a salt formed from the reaction of ammonia and acetic acid. It can be useful for applications that require buffered solutions. One reaction in which ammonium acetate is commonly used in is Henry reactions.
Common Uses:
Basic catalyst for Henry reactions
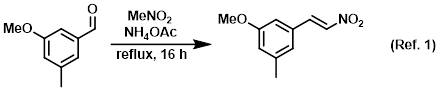
Procedure excerpt:
A mixture of the SM (150 g, 0.999 mol) and NH4OAc (30.8 g, 0.40 mol) in nitromethane (1.5 L) was refluxed for 16 h. The mixture was concentrated then . . .
Safety:
Ammonium acetate is labeled as an irritant.
References:
1) Patent Reference: WO2016014463, page 94, ![]() (6.7 MB)
(6.7 MB)
2) Wikipedia: Ammonium acetate (link)
3) www.sigmaaldrich.com: Ammonium acetate (link)
4) www.alfa.com: A16343 Ammonium acetate, 97% (link)
