Iodination
(NIS)
Examples:
Example 1
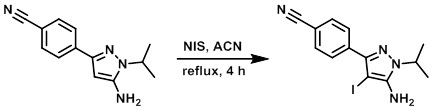
To a solution of the SM (1.45 g, 6.41 mmol) in ACN (26 mL) was added N-Iodosuccimide (1.73 g, 7.69 mmol). The mixture was refluxed for 4 h and then allowed to stir at RT for 16 h. The reaction mixture was then concentrated in vacuo and the crude residue partitioned between H2O (50 mL) and EtOAc (3 x 50 mL). The combined organics were dried (MgSO4), filtered, and concentrated to give a crude solid. The crude was purified by flash chromatography (eluting with 30% EtOAc/pentane) to provide the product as a solid. [1.91 g, 85%] [WO2010032200]
Example 2

A mixture of the SM (2.00 g, 8.09 mmol) and p-toluenesulfonic acid monohydrate (1.50 g, 7.89 mmol) in dry ACN (20 mL) was stirred for 10 min at RT, after which time the mixture was treated with N-Iodosuccinimide (1.82 g, 8.09 mmol) under vigorous stirring. After 2.5 h, the mixture was slowly poured into a cold solution of NaHCO3 (2.0 g) in 50 mL H2O. The resulting solid was filtered, washed with H2O (50 mL), and dried under vacuum overnight to provide the product as a brown solid. [2.35 g, 78%] [WO2012112946, page 206]
Example 3
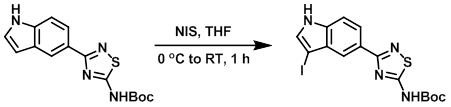
To a solution of the SM (100 mg, 0.316 mmol) in THF (5 mL) at 0 C was added N-Iodosuccinimide (78 mg, 0.348 mmol). The reaction was stirred at RT for 1 h, after which time the mixture was treated with sat aq NaHSO3 (5 mL). The volatiles were removed in vacuo and the crude residue was treated with ice-cold H2O and petroleum ether to provide the product as an off-white solid. [80 mg, 59%] [WO2012129338, page 187]
Example 4

To the SM (1.77 Kg, 8.58 mol) in DMF (8 L) under N2 at 10 C was added N-Iodosuccinimide (1.93 Kg, 8.58 mol) in portions over 10 min. The reaction mixture was stirred at RT for 2 h, after which time it was cooled using an ice bath and quenched with sat aq Na2CO3 (5 L) and extracted with EtOAc (2 x 15 L). The combined organics were washed with sat aq Na2CO3 (2 x 5 L), H2O (3 x 2 L), dried (Na2SO4), and concentrated in vacuo. The resulting crude material was purified by silica gel chromatography (25-40% EtOAc/hexanes) to provide the product. [1.47 Kg, 57% over 2 steps] [WO2012129344, page 123]
