Sodium Bicarbonate
Other Names:
Sodium hydrogen carbonate
General Information:
Structure:
![]()
CAS Number: 144-55-8
Molecular Weight: 84.01 g/mol
Appearance: White crystalline solid
Chemical Formula: NaHCO3
Acidity (pKa): 10.3
Sodium bicarbonate (NaHCO3) is commonly referred to as baking soda by people outside of chemistry. It is amphoteric, meaning it can neutralize both acids and bases. The most common use of NaHCO3 is as a saturated aqueous solution for performing a basic wash during an aqueous workup.
Common Uses:
Basic wash during workups (usually as saturated aq NaHCO3)
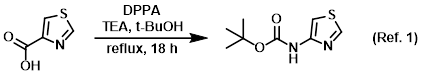
Procedure excerpt:
. . . The residue was diluted with EtOAc (500 mL), washed with H2O (500 mL), sat aq NaHCO3 (500 mL), dried (Na2SO4), and concentrated . . .
Safety:
Sodium bicarbonate is a relatively safe substance. For example, it is safely used in the food and medical industry for various applications. In chemistry, the main safety issues are when using sodium bicarbonate to neutralize acids. Like many acid/base neutralizations it can be an exothermic process. It is also a gas forming reaction. Formation of gas requires that the neutralization be done slowly otherwise the mixture can foam out (or volcano out) of its container.
References:
1) Patent Reference: WO2014201173, page 231, ![]() (19.7 MB)
(19.7 MB)
2) Wikipedia: Sodium bicarbonate (link)
3) www.sigmaaldrich.com : Sodium bicarbonate (link)
