Sodium Sulfate
Other Names:
disodium sulfate
General Information:
Structure:
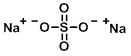
CAS Number: 7757-82-6
Molecular Weight: 142.04 g/mol
Appearance: White crystalline solid
Chemical Formula: Na2SO4
Anhydrous sodium sulfate (Na2SO4) is typically used in organic chemistry as a drying agent. After aqueous extractions the organic layer always has a certain amount of water left in it. Adding anhydrous sodium sulfate removes this water by forming the sodium sulfate hydrate, which conveniently is also a solid allowing it to be filtered away. Magnesium sulfate (MgSO4) is a similar drying agent.
Common Uses:
Drying agent for organic solvents (removes H2O)
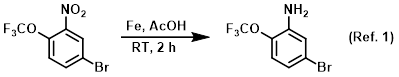
Procedure excerpt:
. . . The mixture was extracted with EtOAc (2 x 50 mL), dried (Na2SO4), and concentrated to provide the product as a brown gummy solid . . .
Saturated aq sodium sulfate solutions are sometimes used to quench LiAlH4 reactions
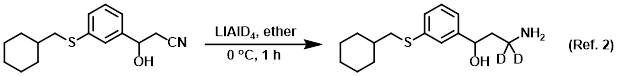
Procedure excerpt:
. . . The reaction was stirred for 1 h, after which time was slowly added sat aq Na2SO4. The mixture was stirred until a precipitate formed, then . . .
Safety:
Sodium sulfate (Na2SO4) is relatively non-toxic. Na2SO4 may cause irritation.
References:
1) Patent Reference: WO2014149164, page 280, ![]() (23.7 MB)
(23.7 MB)
2) UK Pat App GB2463151A, page 153
3) Wikipedia: Sodium sulfate (link)
4) www.sigmaaldrich.com: Sodium sulfate (link)
