Sandmeyer
(Ar-NH2 to Ar-Cl)
Examples:
Example 1
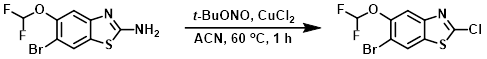
To a suspension of CuCl2 (450 mg, 3.4 mmol) in ACN (8 mL) was added t-BuONO (0.52 mL, 3.9 mmol). The mixture was stirred for 5 min then a solution of the SM (830 mg, 2.8 mmol) in ACN (8 mL) was added dropwise. The reaction mixture was stirred at 60 C for 1 h. After allowing to cool to RT, the mixture was concentrated onto silica gel and purified by silica gel chromatography (0-40% EtOAc/hexane) to provide the product as a light brown powder. [450 mg, 51%]
[Patent Reference: WO2015105749, page 97, ![]() (11.4 MB)]
(11.4 MB)]
Example 2

To a suspension of CuCl (103 mg, 1.04 mmol) in ACN (5 mL) was added t-BuONO (0.134 mL, 1.125 mmol) dropwise at 0 C. A solution of the SM (200 mg, 0.749 mmol) in ACN (4 mL) was added dropwise to the above mixture at 0 C and stirred at the same temperature for 5 min. The reaction mixture was stirred at RT for 30 min, then 70 C for 30 min. The mixture was quenched with H2O (10 mL) and extracted with EtOAc (3 x 10 mL). The combined organics were washed with H2O, dried (Na2SO4), and concentrated in vacuo. The residue was purified by silica gel column chromatography (40% EtOAc/hexane) to provide the product as a brown semi-solid. [68 mg, 31%]
[Patent Reference: WO2015129926, page 190, ![]() (21.5 MB)]
(21.5 MB)]
