TBAF
(Tetra-n-butylammonium Fluoride)
General Information:
Structure:
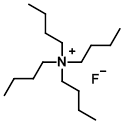
CAS Number: 87749-50-6 (trihydrate)
Molecular Weight: 261.46 g/mol (anhydrous basis)
315.51 (trihydrate)
TBAF is a quaternary salt that is used as a source of fluorine. The fluorine anion is typically used for deprotection of silyl ether groups or as a mild base. The good solubility of TBAF in organic solvents makes it a useful alternative to poorly soluble inorganic bases. Reactions involving TBAF are typically carried out at temperatures below 100 C due to its low thermal stability.
TBAF is typically obtained as a trihydrate (TBAF-3H2O), 1M solution in THF, or 75 wt% solution in H2O. TBAF will always contain some water due to the fact that the fluorine ion is such a great hydrogen bond acceptor.
Common Uses:
Reagent for the SEM deprotection
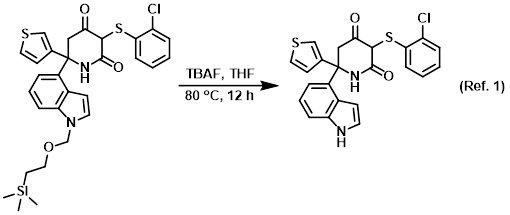
Procedure excerpt:
To a stirred solution of the SM (250 mg, 0.43 mmol) in THF (4 mL) was added TBAF (4 mL, 1M in THF). The reaction mixture was stirred at 80 C for 12 h. After cooling . . .
Reagent for TBS deprotection
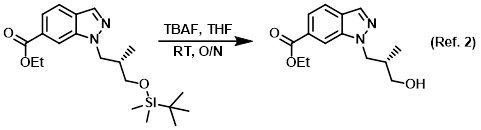
Procedure excerpt:
A mixture of the SM (319 mg, 0.848 mmol) and TBAF (221 mg, 0.848 mmol) in THF (20 mL) was stirred at RT overnight. Upon completion, the resulting solution was . . .
Reagent in substitution reactions with TMSCN
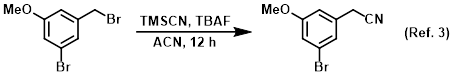
Procedure excerpt:
A solution of the SM (80.0 g, 286 mmol) and TMSCN (28.2 g, 286 mmol) in ACN (600 mL) was stirred at RT for 30 min. The mixture was cooled to 0 C and treated with TBAF . . .
Reagent in SNAr reactions with TMSCN
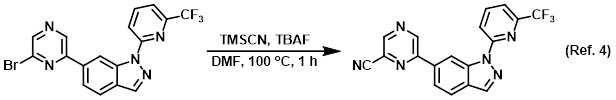
Procedure excerpt:
A mixture of the SM (150 mg, 0.36 mmol), TBAF (281 mg, 1.08 mmol), TMSCN (107 mg, 1.08 mmol) in DMF (3 mL) was stirred at 100 C for 1 h. After cooling . . .
Safety:
TBAF is very hygroscopic. Contact of TBAF with acid liberates toxic gas.
References:
1) Patent Reference: WO2015140133, page 103, ![]() (11.7 MB)
(11.7 MB)
2) Patent Reference: WO2014152144, page 59, ![]() (4.6 MB)
(4.6 MB)
3) Patent Reference: WO2016014463, page 101, ![]() (6.7 MB)
(6.7 MB)
4) Patent Reference: WO2016011390, page 151, ![]() (20.2 MB)
(20.2 MB)
5) Wikipedia: Tetra-n-butylammonium fluoride (link)
6) www.sigmaaldrich.com: Tetrabutylammonium fluoride solution (link)
7) Pearson, A. J.; Roush, W. R.; Handbook of Reagents for Organic Synthesis, Activating Agents and Protecting Groups
