Hydrazine
General Information:
Structure:
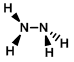
CAS Number: 302-01-2
Molecular Weight: 32.04 g/mol
Appearance: Colorless liquid
Melting Point: 1.4 C
Boiling Point: 113.5 C
Density: 1.021 g/mL
Basicity (pKb): 5.90
Hydrazine is a highly toxic liquid with numerous applications in organic chemistry. It has a pungent ammonia-like odor and is highly flammable. It can be purchased in anhydrous form or as the hydrate. Hydrazine is classified as a strong reducing agent and will react readily and exothermically with most oxidizing agents and mineral acids.
Common Uses:
Reagent in Wolff-Kishner reactions
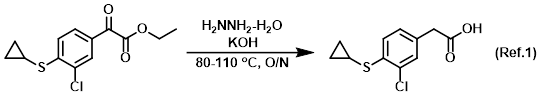
Procedure excerpt:
A mixture of the SM (19.3 g, 75 mmoles) and hydrazine hydrate (18.8 g, 376 mmoles) was heated to 80 C. To the resulting suspension was added KOH (3.37 g, 51 mmoles) and . . .
Reagent for the deprotection of pthalimides
Safety:
Hydrazine is highly toxic and is considered to be dangerously unstable in anhydrous form. Hydrazine is a flammable liquid. Mixtures of hydrazine vapor in the air are flammable between 4.7% and 100% hydrazine by volume. Water solutions of hydrazines cannot be ignited when the hydrazine concentration is below 40%.
References:
1) US20080293730, page 8
2) Wikipedia: Hydrazine (link)
3) www.sigmaaldrich.com: Hydrazine (link)
4) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
5) 22nd Department of Defense Explosives Safety Seminar (link)
