CF3 Acetyl Protection
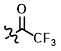
Common Conditions (Protection):
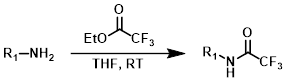
Ethyl Trifluoroacetate
Ethyl trifluoroacetate is convenient for CF3-Acetyl protection because reactions can simply be run in an appropriate solvent (ex. THF) at mild temperatures with no base. The product can be isolated by simply concentrating to remove solvent and the EtOH by-pdt.[1]
Common Conditions (Deprotection):
K2CO3
CF3-Acetyl groups are very labile to basic hydrolysis and can typically be removed under mild conditions such as K2CO3 in aqueous MeOH at RT.[1]
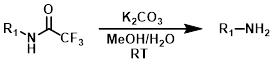
References:
1) Kocienski, P. J.; Protecting Groups, 3rd Edition
