Cesium Carbonate
Other Names:
Caesium carbonate
Carbonic acid dicesium
General Information:
Structure:
![]()
CAS Number: 534-17-8
Molecular Weight: 325.82 g/mol
Appearance: White powder
Chemical Formula: Cs2CO3
Melting Point: 610 C (decomposes)
One of the biggest advantages of cesium carbonate (Cs2CO3) is that it has a higher solubility in most solvents than K2CO3 or Na2CO3.
Common Uses:
Base for substitution reactions
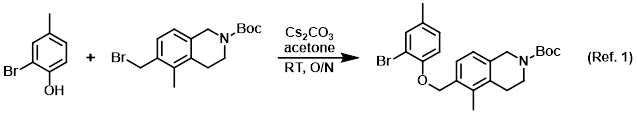
Procedure excerpt:
To a solution of the phenol (0.200 g, 1.07 mmol) in acetone (10 mL) was added the alkyl bromide (0.40 g, 1.2 mmol) followed by Cs2CO3 (1.0 g, 3.1 mmol). The mixture . . .
Base for SNAr reactions
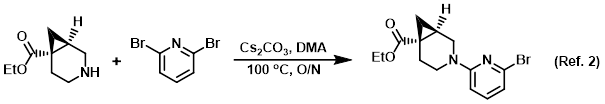
Procedure excerpt:
To a solution of the amine (2.01 g, 11.8 mmol) in DMA (30 mL) was added the aryl bromide (3.65 g, 15.4 mmol), then Cs2CO3 (8.11 g, 24.9 mmol). The reaction . . .
Base for palladium catalyzed reactions (ex. Suzuki reactions)
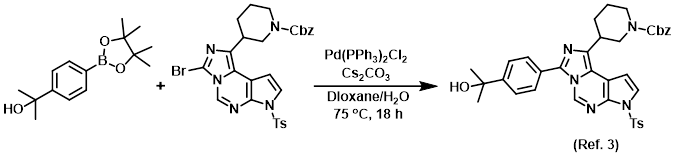
Procedure excerpt:
. . . and dioxane (1.6 mL) were combined to give a yellow suspension. A solution of Cs2CO3 (0.134 g, 0.411 mmol) in H2O (0.4 mL) was added, then N2 was bubbled through . . .
Reagent/base for TBS deprotections
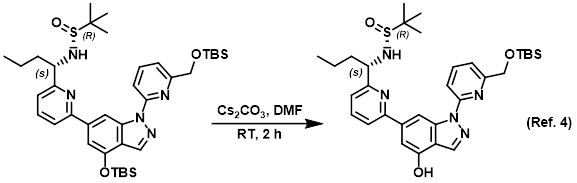
Procedure excerpt:
A mixture of the SM (800 mg, 1.11 mmol) and Cs2CO3 (721 mg, 2.22 mmol) in DMF (10 mL) was stirred at RT for 2 h. The mixture was diluted with H2O (60 mL) and . . .
References:
1) Patent Reference: WO2016014463, page 169, ![]() (6.7 MB)
(6.7 MB)
2) Patent Reference: WO2016014463, page 75, ![]() (6.7 MB)
(6.7 MB)
3) Patent Reference: WO2012149280, page 80, ![]() (4.1 MB)
(4.1 MB)
4) Patent Reference: WO2016011390, page 274, ![]() (20.2 MB)
(20.2 MB)
5) Wikipedia: Caesium carbonate (link)
6) www.sigmaaldrich.com: Cesium carbonate (link)
