Nitro Reduction
![]()
Common Conditions:
H2 + Pd/C
Catalytic hydrogenation with palladium on carbon (Pd/C) is often the method of choice for nitro reductions. Both aromatic and aliphatic nitro groups are reduced to amines. One drawback of H2 + Pd/C is its ability to react with a wide variety of other functionalities on a substrate.[1][2]
![]()
H2 + Raney Nickel
Catalytic hydrogenation with Raney nickel effectively reduces nitro groups. Raney nickel is often used in place of Pd/C for substrates where dehalogenation of aromatic halides (I, Br, and Cl) is a concern.[3]
![]()
Fe
The use of iron (Fe) under acidic conditions (ex. AcOH) provides a mild method for reducing nitro groups to amines in the presence of other reducible groups.
![]()
Zn
The use of zinc (Zn) under acidic conditions (ex. AcOH) provides a mild method for reducing nitro groups to amines in the presence of other reducible groups.
![]()
SnCl2
The use of tin(II) chloride (SnCl2) provides a mild method for reducing nitro groups to amines in the presence of other reducible groups.
![]()
Na2S
Sodium sulfide (Na2S) can be a useful altenative for substrates where hydrogenation or acidic conditions are not compatible. Na2S can sometimes selectively reduce one nitro group in the presence of other nitro groups. Na2S generally does not reduce aliphatic nitro groups.[3][4]

LiAlH4
Lithium aluminum hydride (LiAlH4) reduces aliphatic nitro compounds to amines, but aromatic nitro compounds produce azo products. LiAlH4 is a common reagent for the reduction of nitroalkenes that have been formed using Henry reactions.[2][3]
![]()
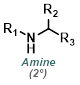
Reaction Map:
The reaction map is intended to provide insight into possible reactions one step before and after the title reaction. It also serves as an alternative way to navigate the website, and as a means of coming up with retrosynthetic ideas. Click on the reaction arrow to visit the page.
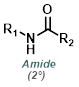 |
||||
 |
||||
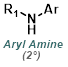 |
||||
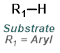 |
 |
 |
||
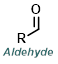 |
 |
|||
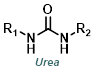 |
||||
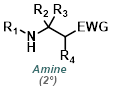 |
||||
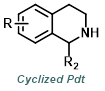 |
References:
1) Caron, S.; Practical Synthetic Organic Chemistry
2) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
3) Smith, M. B.; March's Advanced Organic Chemistry, 7th Edition
4) Hartman, W. W.; Silloway, H. L.; Org. Synth. ; 1945, 25, 5 (link)
