Amine to Amide
(T3P)
Mechanism:
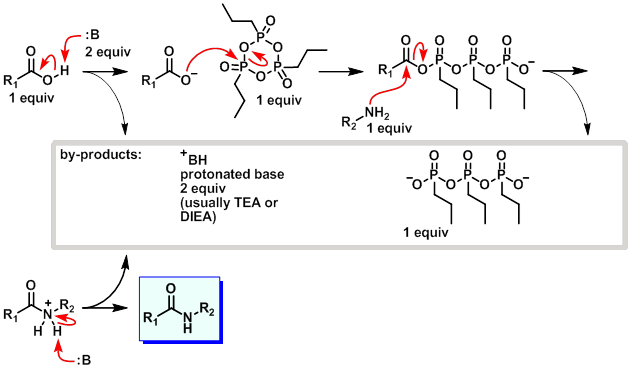
Steps:
- The base deprotonates the carboxylic acid.
- The resulting carboxylate attacks the T3P.
- The amine attacks the now activated carboxylic acid derived intermediate.
- A second equivalent of base picks up the excess proton to provide the amide.
Key Points:
- Base is needed for the rxn to proceed.
- The resulting T3P by-pdt is easily removed by aqueous work-up (usually two H2O washes is enough). The by-pdt can poison downstream rxns involving a catalyst (i.e. Pd catalyzed rxns) so care should be taken to remove most of it.
