Borane Tetrahydrofuran
Other Names:
BH3-THF
General Information:
Structure:
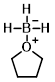
CAS Number: 14044-65-6
Molecular Weight: 85.94 g/mol
Borane-tetrahydrofuran (BH3-THF) is a complex of borane with tetrahydrofuran, and is generally purchased as a solution in THF. BH3-THF can decompose violently, therefore BH3-THF is typically only available in 1 M concentration. It is recommended that BH3-THF solutions be stored at 0-5 C, and that reactions are performed at under 35 C. Borane-dimethylsulfide (BH3-SMe2) is more stable than BH3-THF and is available in much higher concentrations (10 M).
Common Uses:
Reagent for the reduction of carboxylic acids to alcohols
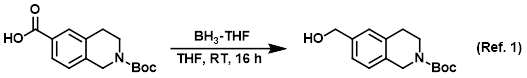
Procedure excerpt:
To a solution of the SM (12.50 g, 45.08 mmol) in dry THF (125 mL) under N2 at RT was added via syringe BH3-THF (99.17 mL, 99.17 mmol). The reaction mixture was . . .
Reagent for the reduction of nitriles to amines
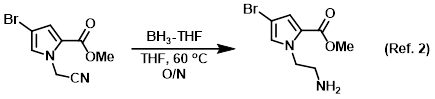
Procedure excerpt:
To a solution of the SM (242 mg, 1 mmol) in THF (10 mL) at RT was added dropwise BH3-THF complex (1.0 M in THF, 5 mL). The reaction was stirred at 60 C . . .
Safety:
The decomposition of BH3-THF generates H2 and B(On-Bu)3. It is recommend that BH3-THF solutions be stored at 0-5 C, and that reactions are performed at under 35 C. BH3-THF in THF is a flammable liquid.
References:
1) Patent Reference: WO2016014463, page 88, ![]() (6.7 MB)
(6.7 MB)
2) Patent Reference: WO2014149164, page 315, ![]() (23.7 MB)
(23.7 MB)
3) Wikipedia: Borane–tetrahydrofuran (link)
4) www.sigmaaldrich.com: Borane tetrahydrofuran complex solution (link)
5) Anderson, N. G.; Practical Process Research and Development, a Guide for Organic Chemists, 2nd Edition
6) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
